(NEW YORK) — The first oral pill for obstructive sleep apnea (OSA) could be around the corner after pharmaceutical company Apnimed Inc. reported positive results from its stage III clinical trial.
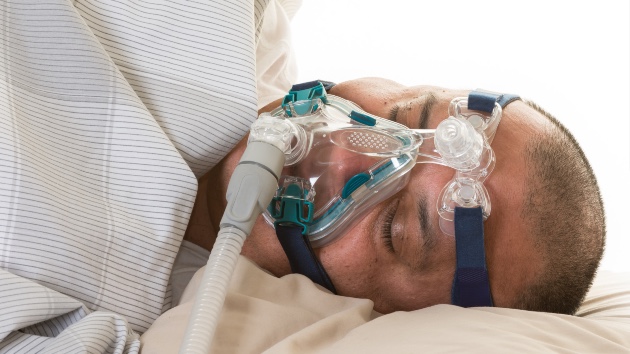
Currently, many people diagnosed with OSA patients require a machine that covers their nose or both the nose and mouth during sleep and delivers air through a mask to help keep their airways open.
Apnimed’s lead candidate AD109 showed “clinically meaningful and statistically significant reductions” in airway obstruction after 26 weeks, the company said in a press release.
AD109, a once-a-day pill, is a neuromuscular modulator that increases upper airway muscle tone, which is how contracted the muscles are in the upper airway.
OSA patients treated with the medication saw a nearly 50% reduction in the severity from baseline at week 26, compared to 6.8% of those in the placebo group.
The reduction was “significant” at the end of the study period, which concluded at 51 weeks. At the end of the trial, nearly 23% of participants saw “complete disease control.”
The results were part of Apnimed’s 12-month study looking at the safety and efficacy of AD109 in adults with mild, moderate and severe OSA.
AD109 was well-tolerated among participants with only mild or moderate adverse events. Which was consistent with prior studies, according to Apnimed. No serious adverse events were reported in the trial.
“With two large Phase 3 studies now demonstrating a consistent and significant efficacy profile for AD109, we are closer to delivering the first oral pharmacotherapy for over 80 million U.S. adults with OSA,” Dr. Larry Miller, CEO of Apnimed, said in a statement. “Given the scale of unmet need in OSA, where the majority of patients remain untreated, we believe AD109, as a simple once-daily oral drug, has the potential to expand and reshape the treatment landscape, which would represent a significant commercial opportunity for Apnimed.”
OSA is a sleep disorder in which the airways become narrowed or blocked while sleeping, causing breathing to pause, according to MedlinePlus.
Soon after falling asleep, people experience loud and heavy snoring. The snoring is often interrupted by a long silent period during which breathing stops and then followed by a loud snort and gasp as the patient attempts to breathe.
This can cause excessive daytime sleepiness and affect quality of life, mental well-being and cardiovascular health.
In addition to a CPAP machine, there are lifestyle changes that people with sleep apnea can make including avoiding alcohol or medications that cause drowsiness and losing excess weight.
Recently, the U.S. Food and Drug Administration (FDA) expanded approval of Eli Lilly’s obesity medication Zepbound to include treating moderate to severe obstructive sleep apnea for people with obesity.
The clinical trial did examine patients with a wide range of “weight classes” and did not see differences in efficacy based on weight.
Apnimed plans to file a New Drug Application with the FDA in early 2026, according to Miller.
Copyright © 2025, ABC Audio. All rights reserved.